Abstract
The balance between hemostasis and thrombosis is critical to human health. However, intensified thrombosis that breaks this balance is often observed in diseases like hypertension, diabetes and obesity, leading to atherothrombosis in the coronary and cerebral circulations [1]. Current therapeutic strategies to reduce thrombotic risk are efficacious, as they have reduced morbidity and mortality, but still suboptimal, as commercialized drugs carry the risk of excessive bleeding. A new generation of drugs targeted to increase or maintain antithrombotic efficacy while reducing the negative impact on hemostasis is needed.
The formation of platelet aggregates over an arterial vascular injury site is a two-step process. Firstly, binding between platelet glycoprotein (GP) Ibα - a component of the GPIb-IX-V receptor complex - and subendothelial von Willebrand Factor (VWF) at its A1 domain (VWFA1) enables recruitment of circulating platelets under high shear force. This represents the first layer of the hemostatic plug initiated by VWFA1-GPIb and consolidated by adhesion of activated integrins. Secondly, adhesion receptors on the up-facing side of the attached platelets engage circulating proteins including VWF, fibrinogen and fibronectin, among others, attracting new platelets into the growing thrombus. Importantly, this step is shared by atherothrombosis as a mechanism of thrombus growth. The GPIbα-VWFA1 interaction operates in both steps, which respectively mediate platelet adhesion and thrombogenesis [2]. Notably, when VWF is immobilized, its association with platelet GPIbα is mediated by two-dimensional (2D) kinetics; in contrast, the attachment of plasma VWF to immobilized platelets in the growing aggregate is governed by three-dimensional (3D) kinetics.
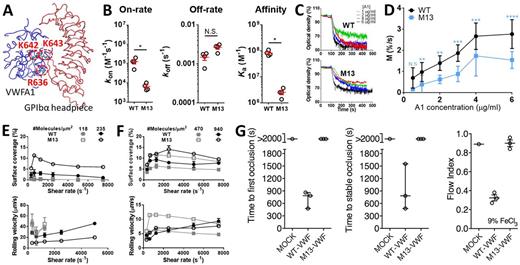
We have identified positively charged, GPIbα-non-contact residues in helices α4/α5 of the VWFA1 that partially affect the VWF-GPIbα interaction. A triple mutation, R636/K642/K643A (referred to as M13) (Fig. 1A) drastically reduces the 3D on-rate of binding to GPIbα without significantly affecting the off-rate, which remains comparable to VWFA1-WT as measured by surface plasma resonance. In this assay, purified GPIbα molecules were immobilized on a surface that was then perfused with soluble monomeric VWFA1 at different concentrations. As a consequence of the decreased 3D on-rate, the 3D GPIbα binding affinity of M13 VWFA1 is 20-fold lower than that of WT VWFA1 (Fig. 1B). In agreement with this finding, aggregometry experiments showed that platelet agglutination mediated by dimeric M13 VWFA1 in the presence of ristocetin was greatly attenuated compared to WT (Fig. 1C), and the speed of agglutination was also slowed (Fig. 1D), implying that M13 is weaker in establishing platelet-platelet interactions.
In contrast, the M13 mutation does not compromise the adhesion of platelets to immobilized VWFA1. In a flow chamber system, anticoagulated whole blood was perfused over a surface pre-coated with monomeric WT or M13 VWFA1. Under a range of shear rates and with different VWFA1 coating densities, the coverage of platelets over immobilized M13 VWFA1 was never less, if not greater than on WT VWFA1 (Fig. 1E,F, upper panels); accordingly, the rolling velocity of adherent platelets was similar or slower on M13 than WT VWFA1 (Fig. 1E,F, lower panels).
We next investigated how the VWF M13 mutation affects in vivo thrombogenesis using a VWF-/- mouse strain in which platelets expresses human GPIbα. Mammalian cell expressed full-length human WT and M13 VWF was infused into these mice in combination with human FVIII to correct for the coagulation associated with VWFdeficiency; a carotid artery lesion was then caused with 9% ferric chloride. Our preliminary results showed that WT VWF restored a complete thrombotic occlusion of the artery, but mutant M13 VWF was without effect (Fig. 1G).
Overall, our data suggests that VWFA1 domain can be engineered to support efficient platelet adhesion, which is key to hemostasis, while being less prone to mediate endovascular thrombogenesis. The development of this study should provide new insights into the distinct mechanisms that support VWF contributing to hemostasis or thrombosis, as well as into designing more selective pro-hemostatic VWF molecules with a reduced risk of causing thrombotic complications.
References:
[1] S.P. Jackson, Nat Med 17(11) (2011) 1423-36.
[2] Z.M. Ruggeri, JTH 1(7) (2003) 1335-42.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal